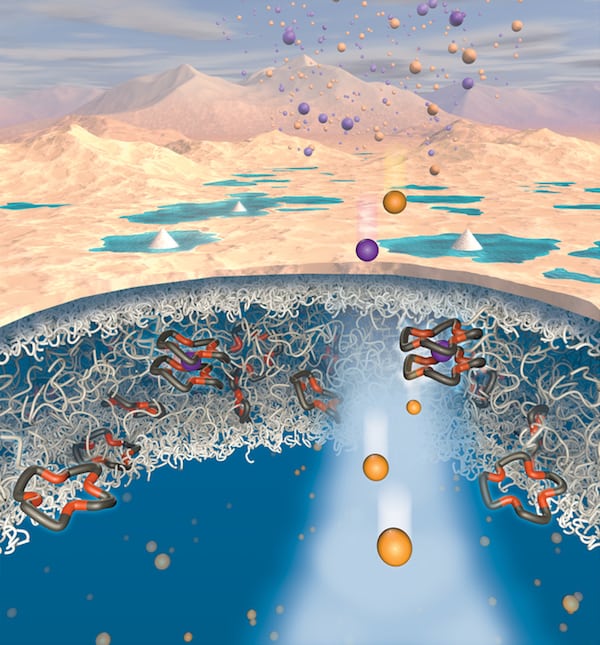
A new way to extract lithium from contaminated water could make this technologically important metal much easier to produce. The technique, which involves passing aqueous brines through lithium-selective polymeric membranes, works in a way that mimics the potassium channels that regulate the balance of ions in biological systems.
Lithium has several applications in low-carbon energy and is widely employed in electrochemical technologies. Lithium-ion batteries, for example, dominate today’s market for rechargeable power storage thanks to the element’s low mass, large reduction potential and high energy density.
As electric vehicles become more popular, industrial demand for lithium is set to increase still further. This creates challenges, because although lithium is an earth-abundant metal, extracting it from natural sources is not easy. Currently, it is sourced from deposits of a mineral called pegmatite and salt brines via solar evaporation – a costly and inefficient process that can take over a year.
Crown ethers
Researchers have previously explored ways of using polymer membranes to extract lithium from aqueous solutions. Conventional polymer membranes typically separate solutes based on differences in either the size or the charge of ions, but this is not specific enough to target lithium alone. Most such membranes allow sodium ions to permeate at a greater rate than lithium ones.
A team led by Benny Freeman of the University of Texas at Austin has now succeeded in reversing this behaviour by developing a novel polymer membrane containing crown ethers – chemically functionalized ligands that can bind certain ions. These ligands hinder the permeation of sodium but “ignore” lithium, meaning it passes through the membrane at a greater rate than sodium. Indeed, the team’s lithium transport measurements revealed that the material boasts an unprecedented reverse permeability selectivity, preferring lithium over sodium by a factor of roughly 2.3 – the highest selectivity ever documented for a dense, water-swollen polymer.
“Lithium is currently extracted from brines through the use of evaporation ponds, which is a slow and laborious process,” explains Freeman. “Using membranes such as ours that can extract lithium is advantageous because they are energy efficient, scalable and can have a much higher throughput than evaporation ponds.”
Solute specific selectivity
By tuning a polymer’s interactions, it is possible to make the material’s selectivity specific to the desired solute, he tells Physics World. “Such selectivity is observed in biological systems, such as potassium channels, which motivated the design of our material system.”
Process innovations benefit battery manufacture
As well as lithium extraction, the researchers say the new membranes might also be useful for removing toxic solutes from water. Further ahead, the team plan to study the factors that affect the “host-guest” interaction between solutes and the polymer membranes at the molecular scale. “These studies will include looking into the polymer structure as well as identifying new ligands for making the membrane more selective to lithium,” Freeman says.
The research is detailed in PNAS.




